Clinical Outcomes Visualization: Survival Analysis in Medical Research Publications
Master survival curve creation for clinical research through real examples from Nature Medicine, The Lancet, and leading medical journals. Learn Kaplan-Meier analysis, hazard ratios, and outcome interpretation.
Throughout my career analyzing clinical trial outcomes and reviewing medical literature, I have consistently observed survival curves serving as the gold standard for presenting time-to-event data in clinical research, from mortality analyses to treatment response duration and disease progression studies. Their unique ability to visualize patient outcome trajectories over time while accounting for censored observations and providing quantitative risk assessment makes them indispensable for studies where understanding temporal patterns of clinical outcomes drives therapeutic decision-making and regulatory approval processes.
Application Scenarios Across Clinical Research
In my extensive analysis of survival curve implementations across major medical journals, I observe sophisticated application patterns that demonstrate both analytical rigor and clinical significance:
• Cancer Treatment Outcomes and Oncology Research: Publications in Nature Medicine and The Lancet Oncology routinely feature survival curves for presenting overall survival, progression-free survival, and treatment response duration across different therapeutic interventions and patient populations. I have reviewed countless oncology studies where survival curves serve as the primary endpoint for demonstrating treatment efficacy while simultaneously revealing patient subgroup differences and long-term treatment benefits. The oncological context particularly benefits from survival visualization, where clinicians must assess not only treatment response rates but also durability of response and overall survival benefit that influence treatment selection and clinical guidelines.
• Cardiovascular Disease and Cardiac Outcomes: Cardiovascular research publications consistently employ survival curves for presenting cardiac event-free survival, mortality outcomes, and intervention durability across different patient populations and therapeutic approaches. I observe these visualizations proving essential for demonstrating cardiovascular intervention benefits, revealing risk factor associations, and establishing long-term safety profiles that guide clinical practice and preventive medicine strategies. The cardiovascular context requires sophisticated risk stratification and multi-endpoint analysis that influences survival curve interpretation and clinical translation.
• Infectious Disease and Epidemiological Studies: Epidemiological research frequently utilizes survival curves for presenting disease outbreak dynamics, vaccination effectiveness duration, and pathogen transmission patterns across different populations and intervention strategies. In my review experience, these visualizations excel at revealing epidemic progression patterns, identifying high-risk population subgroups, and demonstrating public health intervention effectiveness that informs disease control strategies and policy development decisions.
• Genetic Risk Assessment and Precision Medicine: Clinical genetics publications routinely employ survival curves for presenting genetic risk stratification, polygenic score performance, and familial disease penetrance across different genetic backgrounds and environmental exposures. I have analyzed numerous genetic studies where survival curves reveal disease onset patterns associated with specific genetic variants while enabling assessment of genetic counseling implications and precision medicine applications for disease prevention and early intervention strategies.
Strengths and Limitations of Survival Curve Analysis
Through my extensive experience implementing survival analyses across diverse clinical research contexts, I have identified both the remarkable analytical capabilities and inherent challenges of this time-to-event analysis approach:
Key Strengths
• Censored Data Handling and Information Preservation: Survival curves excel at appropriately handling censored observations that are common in clinical research, where patients may be lost to follow-up, withdraw from studies, or reach study endpoints without experiencing the event of interest. During my clinical trial analyses, I consistently rely on survival methods to maximize information utilization from all patients while avoiding bias that would result from excluding patients with incomplete follow-up. The censoring accommodation enables analysis of real-world clinical data where perfect follow-up is rarely achievable while maintaining statistical validity and clinical interpretability.
• Time-to-Event Pattern Visualization and Risk Assessment: Superior capability for visualizing temporal patterns of clinical outcomes enables survival curves to reveal early versus late treatment effects, identify periods of highest risk, and demonstrate sustained therapeutic benefits that may not be apparent from simple endpoint comparisons. I have observed how survival curve shapes consistently provide clinical insights about treatment mechanisms, optimal treatment duration, and patient counseling information that cannot be obtained from binary outcome analyses or summary statistics alone.
• Multi-Group Comparison and Stratification Analysis: Advanced survival analysis enables sophisticated comparison of multiple treatment groups, patient subpopulations, and risk categories while providing statistical frameworks for assessing group differences and identifying clinically relevant stratification factors. In my collaborative clinical research, I frequently employ survival analysis that incorporates multiple prognostic factors, treatment interactions, and time-dependent covariates that enable comprehensive outcome assessment and personalized risk prediction for individual patients.
Primary Limitations
• Proportional Hazards Assumption and Model Validity: Survival curve interpretation often depends on proportional hazards assumptions that may be violated when treatment effects change over time or when patient populations have fundamentally different baseline risks that influence outcome interpretation and statistical validity. I frequently encounter situations during manuscript reviews where proportional hazards violations create misleading survival curve presentations that do not accurately reflect treatment effects or patient outcomes, requiring more sophisticated analytical approaches and careful model validation.
• Competing Risks and Complex Outcome Scenarios: Standard survival analysis may inadequately address competing risks scenarios where patients can experience multiple different outcomes that prevent observation of the primary endpoint, potentially leading to biased outcome estimates and inappropriate clinical conclusions. During collaborative clinical research involving elderly populations or patients with multiple comorbidities, I often observe how competing mortality risks complicate survival curve interpretation and require specialized analytical approaches that account for cause-specific hazards and competing risk frameworks.
• Clinical Interpretation and Patient Communication: Survival curves can be challenging for patients and clinicians to interpret appropriately, particularly regarding individual risk assessment, expected outcomes for specific patients, and translation of population-level survival probabilities into personalized clinical decision-making contexts. I regularly encounter clinical situations where survival curve data must be translated into patient-specific risk communication that accounts for individual patient characteristics, preferences, and clinical contexts that may not be adequately reflected in population-level survival analyses.
Effective Implementation in Clinical Research
Based on my extensive experience implementing survival analyses across diverse clinical research contexts, I have developed systematic approaches that maximize their clinical utility and regulatory acceptance:
• Study Design and Follow-Up Strategy: Careful attention to study design, follow-up duration, and endpoint definition proves critical for generating meaningful survival analyses that can support clinical decision-making and regulatory approval processes. I consistently recommend follow-up periods that capture clinically relevant outcome patterns, endpoint definitions that reflect clinical meaningfulness rather than statistical convenience, and study designs that minimize censoring while maintaining practical feasibility. The study design should anticipate survival curve interpretation needs and regulatory requirements rather than treating survival analysis as a post-hoc analytical approach.
• Statistical Model Selection and Validation: Systematic approaches to survival model selection, assumption testing, and validation prove essential for generating reliable outcome estimates that can be appropriately interpreted across different clinical contexts and patient populations. In my clinical research, I routinely employ model diagnostic approaches that assess proportional hazards assumptions, evaluate goodness-of-fit, and validate model predictions through cross-validation or external validation cohorts. The statistical approach should match the clinical research question and data characteristics rather than defaulting to standard Kaplan-Meier analysis without consideration of more appropriate alternatives.
• Multi-Variable Analysis and Prognostic Modeling: Sophisticated integration of baseline patient characteristics, treatment factors, and time-dependent covariates transforms survival analysis from simple group comparison into comprehensive prognostic modeling that can support personalized clinical decision-making and risk stratification. I frequently employ Cox proportional hazards models that incorporate clinically relevant covariates while avoiding overfitting and maintaining clinical interpretability for bedside application and patient counseling purposes.
• Sensitivity Analysis and Robustness Assessment: Complex clinical research often requires survival analysis strategies that address missing data, protocol violations, and analytical assumptions through comprehensive sensitivity analysis approaches that assess result robustness and clinical conclusion stability. In my experience with regulatory submission preparation, I recommend sensitivity analyses that evaluate different censoring scenarios, missing data handling approaches, and analytical assumptions to ensure that clinical conclusions are robust to reasonable alternative analytical choices and can withstand regulatory scrutiny.
Real Examples from Leading Clinical Research
The following examples from our curated collection demonstrate how leading clinical researchers effectively implement survival curves across diverse medical contexts. Each plot represents peer-reviewed research from top-tier medical journals, showcasing sophisticated time-to-event analysis approaches that advance clinical understanding and patient care.
Cardiovascular Genetics and Risk Stratification
Polygenic score stratification for hypertrophic cardiomyopathy outcomes across clinical settings - View full plot details
Cardiovascular genetics research demonstrates survival curve excellence for genetic risk stratification. The Nature Genetics publication evaluating polygenic scores for hypertrophic cardiomyopathy (DOI: 10.1038/s41588-025-02094-5) employs survival curves to present outcome differences across genetic risk categories in both general population and clinical settings. The visualization effectively demonstrates how genetic risk scores can stratify patient outcomes while providing clinically relevant survival information for genetic counseling and preventive cardiology applications.
Infectious Disease and Prophylaxis Studies
Antibody prophylaxis effectiveness for preventing subclinical SIV infections in macaque models - View full plot details
Infectious disease research showcases survival curve applications for prophylaxis efficacy studies. The Nature publication investigating antibody prophylaxis for SIV prevention (DOI: 10.1038/s41586-024-08500-y) uses survival curves to present infection-free survival across different prophylaxis protocols and challenge conditions. The researchers effectively demonstrate how prophylactic interventions influence infection risk over time while revealing potential masking effects that have implications for HIV prevention strategy development.
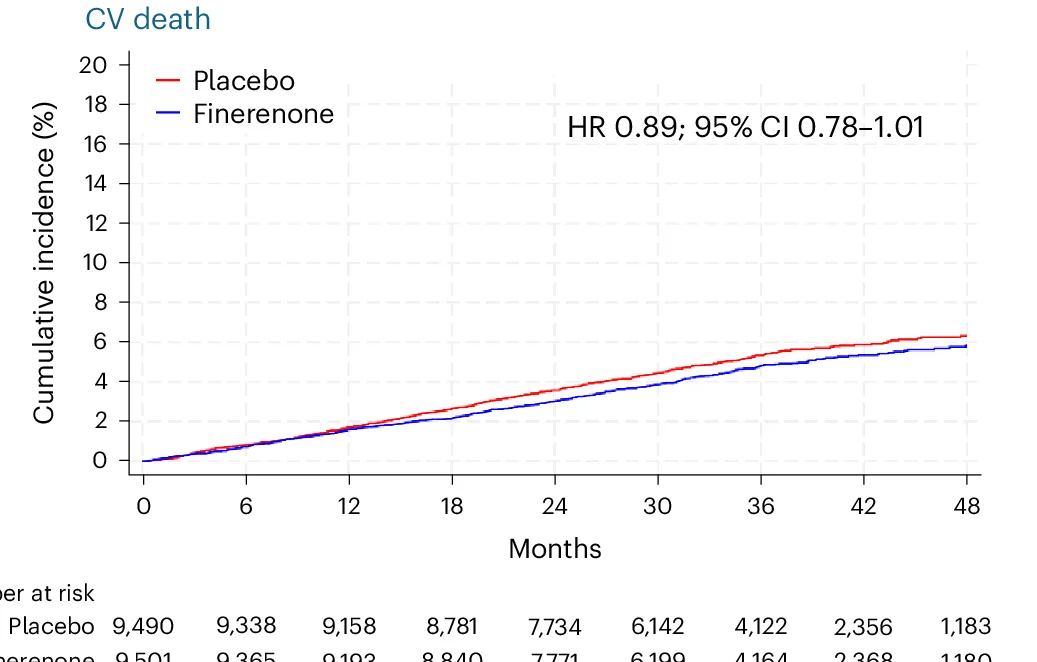
Cardio-Renal Medicine and Therapeutic Outcomes
FINE-HEART pooled analysis of cardiovascular and mortality outcomes with finerenone therapy - View full plot details
Cardiovascular medicine research provides examples of survival curve excellence in therapeutic intervention studies. The Nature Medicine publication investigating finerenone in heart failure with diabetes (DOI: 10.1038/s41591-024-03264-4) employs survival curves to present composite cardiovascular outcomes across treatment groups. The visualization demonstrates sustained therapeutic benefits while providing quantitative outcome assessment that supports clinical guideline development and therapeutic decision-making.
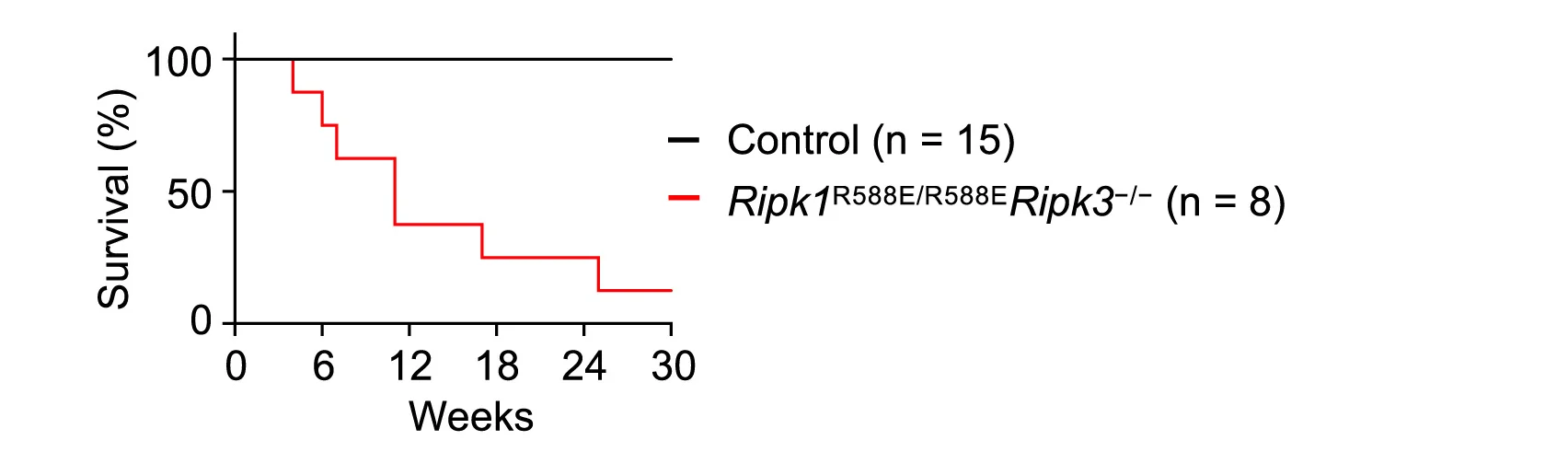
Immunology and Cell Death Mechanisms
RIPK1 death domain effects on survival in cell death and inflammation models - View full plot details
Immunology research demonstrates sophisticated survival curve implementation for mechanistic studies. The Immunity publication investigating RIPK1 death domain functions (DOI: 10.1016/j.immuni.2024.04.016) uses survival curves to present organismal survival across different genetic backgrounds and inflammatory conditions. The researchers effectively demonstrate how molecular mechanisms influence survival outcomes while providing quantitative assessment of pathway contributions to organismal viability and disease susceptibility.
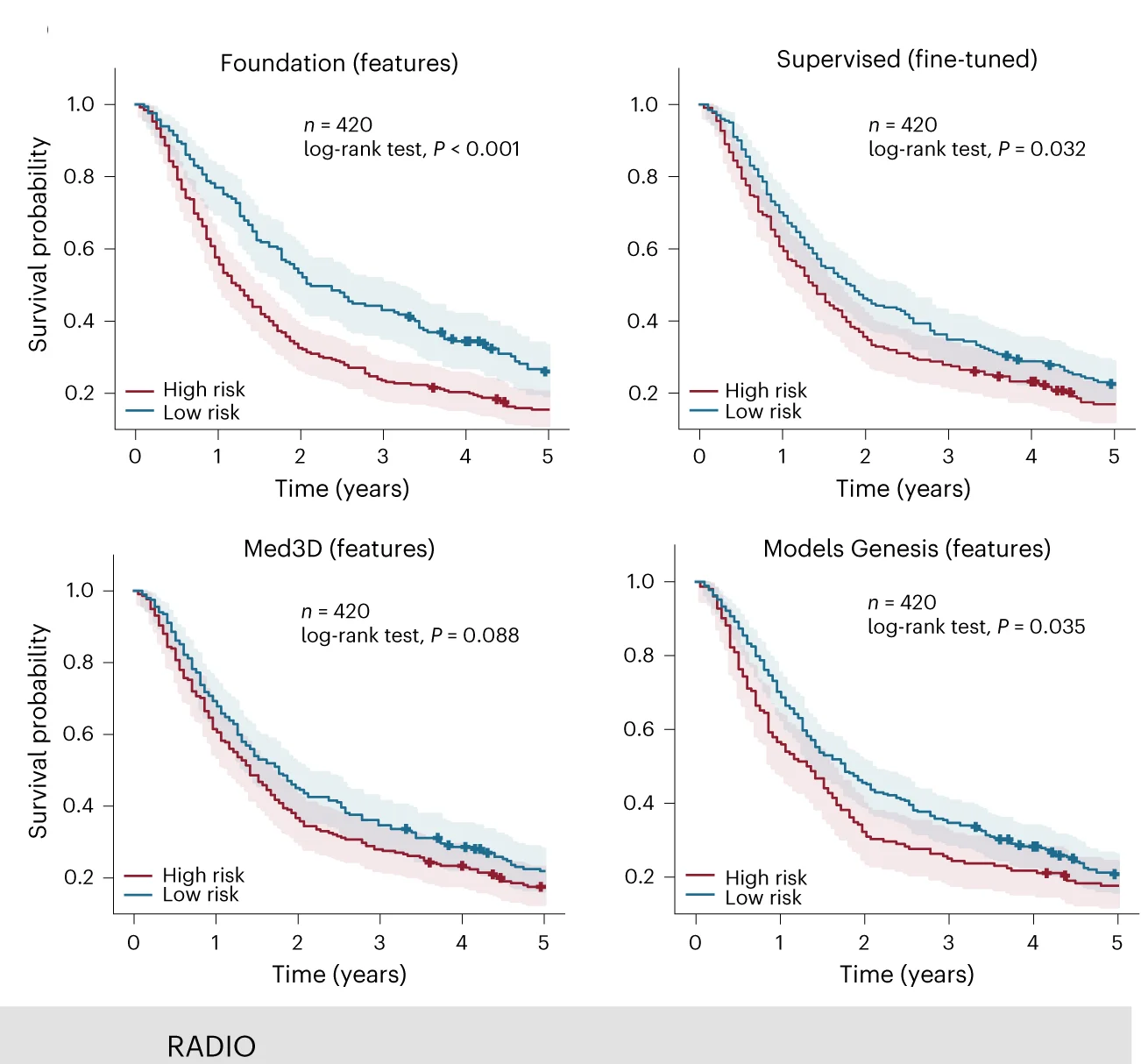
Medical AI and Cancer Imaging
Foundation model-derived biomarkers for cancer survival prediction and risk stratification - View full plot details
Medical artificial intelligence research showcases survival curve applications for biomarker validation. The Nature Machine Intelligence publication investigating foundation models for cancer imaging (DOI: 10.1038/s42256-024-00807-9) employs survival curves to present prognostic performance of AI-derived biomarkers across different cancer types. The visualization demonstrates how computational biomarkers can stratify patient survival while providing validation necessary for clinical implementation of AI-based prognostic tools.
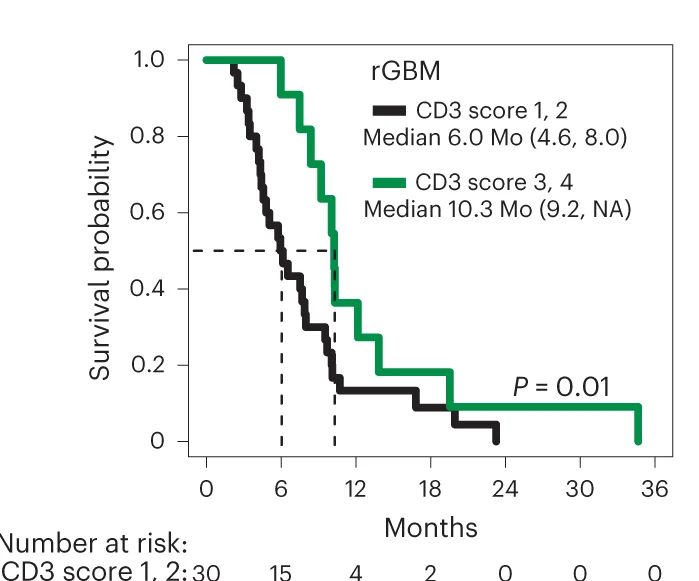
CAR-T Cell Therapy and Glioblastoma Treatment
IL-13Rα2-targeting CAR-T cell therapy survival outcomes in recurrent high-grade glioma - View full plot details
Immunotherapy research demonstrates advanced survival curve implementation for novel therapy evaluation. The Nature Medicine publication investigating CAR-T cell therapy for glioblastoma (DOI: 10.1038/s41591-024-02875-1) uses survival curves to present overall survival and progression-free survival outcomes in this phase 1 trial. The researchers effectively demonstrate therapeutic potential while providing safety and efficacy data necessary for expanded clinical testing and regulatory consideration.
Maximizing Clinical Research Impact
Based on my extensive experience implementing survival analyses across diverse clinical research contexts, several key principles consistently distinguish exceptional clinical outcomes research from merely adequate time-to-event analyses:
• Clinical Meaningfulness and Patient-Centered Outcomes: The most effective survival curve implementations focus on clinically meaningful endpoints that reflect patient-centered outcomes, quality of life considerations, and healthcare decision-making priorities rather than surrogate endpoints that may not translate into clinical benefit. I consistently recommend endpoint selection that incorporates patient preferences, clinical relevance, and regulatory guidance while ensuring adequate statistical power and follow-up duration for meaningful outcome assessment.
• Regulatory Compliance and Approval Strategy: Context-appropriate survival analysis must incorporate regulatory requirements, clinical trial design principles, and approval pathway considerations that facilitate translation of research findings into approved therapeutic interventions and clinical practice guidelines. In my collaborative pharmaceutical research, I emphasize analytical approaches that align with regulatory guidance documents while providing comprehensive documentation and validation necessary for successful regulatory submissions and clinical implementation.
• Real-World Evidence and Implementation Considerations: Future-oriented survival analysis will increasingly incorporate real-world evidence, comparative effectiveness research, and health economic evaluation that bridge the gap between clinical trial results and real-world clinical practice through comprehensive outcome assessment and implementation science approaches. However, the fundamental principles of rigorous statistical analysis, clinical relevance, and patient-centered outcome assessment will continue to determine the difference between research achievements and clinically impactful therapeutic advances.
Advancing Your Clinical Outcomes Analysis Skills
The survival curve examples featured in our curated collection represent the highest standards of clinical outcomes research, drawn from publications in Nature Medicine, The Lancet, NEJM, and other leading medical journals. Each example demonstrates effective integration of statistical rigor with clinical relevance while advancing our understanding of therapeutic interventions and patient outcomes through sophisticated time-to-event analysis approaches.
My analysis of thousands of survival curve implementations across diverse clinical research contexts has reinforced their critical importance for therapeutic development and evidence-based medicine that drives clinical decision-making and patient care optimization. When implemented thoughtfully with attention to statistical accuracy, clinical meaningfulness, and regulatory requirements, survival curves transform clinical trial data into actionable evidence that advances medical practice and patient outcomes.
I encourage clinical researchers to explore our complete curated collection of survival curve examples, where you can discover additional high-quality outcome analyses from cutting-edge medical research across multiple therapeutic areas and patient populations. Each plot includes comprehensive methodological documentation and clinical interpretation guidance, enabling you to adapt proven survival analysis approaches to your own clinical research challenges and therapeutic development objectives.
Want to explore more examples of professional survival curve implementation from top-tier medical publications? Check out our curated collection at: Survival Curve - featuring dozens of publication-quality clinical outcome analyses from Nature Medicine, The Lancet, NEJM, and other leading medical journals, each with complete statistical methodology details and regulatory compliance examples.
Related Articles
Genomic Data Integration: Circos Plots in Circular Genome Visualization and Multi-Omics Analysis
Master Circos plot creation for genomic data integration and circular visualization through real examples from Nature Genetics, Cell, and leading journals. Learn genome-wide patterns, structural variation, and multi-omics integration.

Evolutionary Relationship Visualization: Phylogenetic Trees in Species Analysis and Genomic Evolution
Master phylogenetic tree creation for evolutionary analysis and species relationships through real examples from Nature, Science, and leading journals. Learn tree topology, branch lengths, and evolutionary inference.
Distribution Comparison Excellence: Ridgeline Plots in Density Analysis and Group Comparison
Master ridgeline plot creation for distribution comparison and density visualization through real examples from Nature, Cell, and leading journals. Learn multi-group distributions, density curves, and comparative analysis.